Disordered proteins
While intrinsically disordered proteins can feature a diverse structural ensemble in solution, their highly ordered, extended aggregates often found in the form of amyloid fibrils are a major hallmark of neurodegenerative diseases.
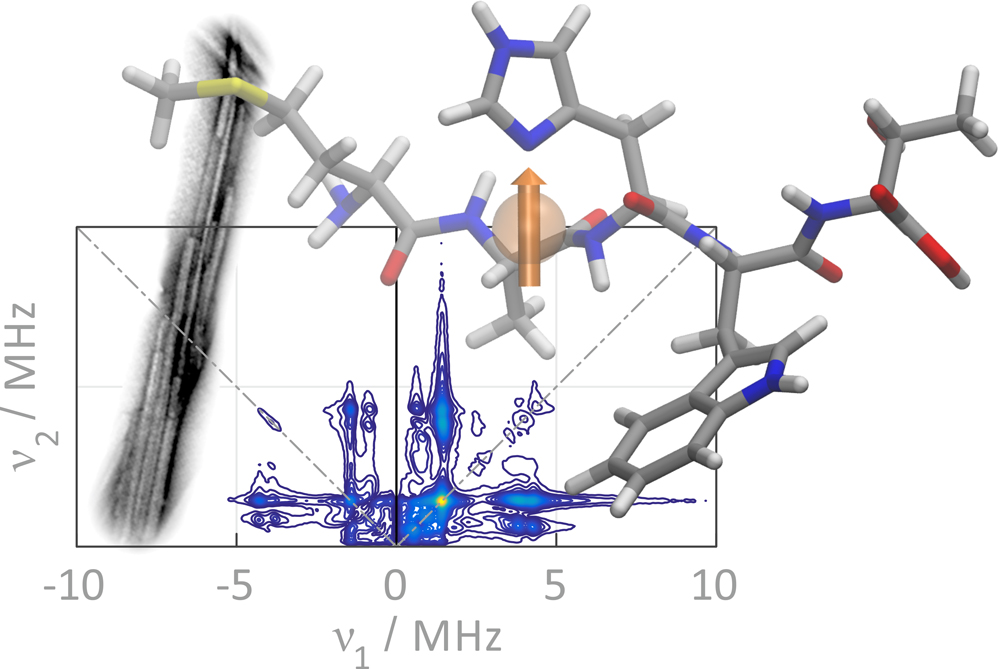
Recent studies suggest links between fibrillation and binding of divalent metal ions, while the metal-facilitated aggregation process and the toxicity of the oligomeric intermediates are currently not fully understood.
We address these questions by using the bound metal ions as spin labels to investigate the metal binding and coordination as well as by using nitroxide spin labels to monitor conformation and dynamics at the different stages of the fibrillation process.